Introduction:
Venous thromboembolic disease (VTE) is a common cause of morbidity and mortality in patients with cancer. Cancer associated thrombosis (CAT) is associated with poor prognosis, worse survival and increased care costs. Previous trials demonstrated the non-inferiority of DOACs to LMWH for the treatment of CAT, but there is concern for a possible increased incidence of bleeding, particularly in patients with gastrointestinal (GI) and urothelial (GU) cancers. Current guidelines suggest a preference for enoxaparin with these diagnoses. The aim of our study is to elucidate the safety of DOACs in the treatment of GI and GU cancer associated thrombosis at our institution.
Methods:
Retrospective chart review of patients with GI or GU cancer associated thrombosis who received either enoxaparin or DOACs (apixaban or rivaroxaban) was performed. The baseline characteristics, duration of anticoagulation (AC) and bleeding events (BEs) were compared between the two groups. The bleeding events were classified according to ISTH categories. Statistical analysis was done using R studio (V1.3.1056).
Results:
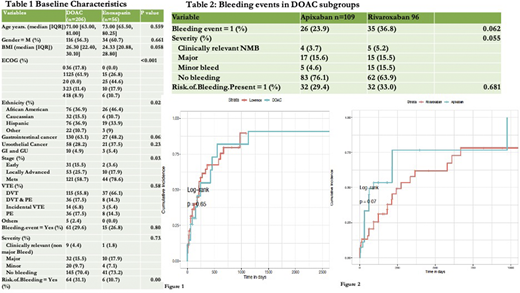
All patients from 01/2001 - 01/2020 with active GI or GU cancer and associated thrombosis who had received enoxaparin or a DOAC were included in the study. Of 262 patients reviewed, 206 (78.6 %) received a DOAC and 56 (21.4%) received enoxaparin. The baseline characteristics between the two groups are depicted in Table 1. Patients in the DOAC group had lower ECOG scores than those on enoxaparin: 79.7% of DOAC and 26.8% of enoxaparin patients had ECOG scores of 1-2. Patients on DOACs were less likely to have metastatic disease (58.7% vs. 78.6%) but were more likely to have additional risk factors for bleeding (p=0.004): 24 patients (11.7%) on DOACs were also on an antiplatelet agent (19 on aspirin, 5 on clopidogrel), compared to 4 patients (7.1%) in enoxaparin group (3 on aspirin, 1 on clopidogrel). Clot distribution was similar between the two groups. The majority of patients, 70.4% in the DOAC group and 73.2% in the enoxaparin group, had no BEs. There was no statistically significant difference in the cumulative incidence (CI) of bleeding between the DOAC and enoxaparin groups (p-value 0.65) Figure 1.
In the DOAC group, 109 patients (52.9%) received apixaban and 97 patients (47.1%) received rivaroxaban. There were 26 (23.9%) and 35 (36.8%) BE in the apixaban and rivaroxaban subgroups respectively, Table 2. Of those on apixaban, 13/26 had GI bleeding (12 had underlying GI and 1 patient had GU cancer) and 10/26 had GU bleeding(all with underlying GU cancer). Of those on rivaroxaban 18/35 had GI bleeding (all with underlying GI cancer) and 11/35 had GU bleeding (6 patients had GU and 5 had GI cancer).
Conclusion:
Our study suggests that physician preferences play a major role in the choice of AC. Most physicians preferred DOACs even for patients with GI/GU cancers when patients had better ECOG scores and non-metastatic disease. However, these were also the same patients that were then more likely to have been exposed to additional risk factors for bleeding. BEs between DOACs and enoxaparin were similar and, between the DOACs, somewhat more favorable with apixaban than rivaroxaban. Randomized clinical trials, controlling for physician choice and bleeding risk factor, are necessary valid comparisons for the best choice of anticoagulation.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal